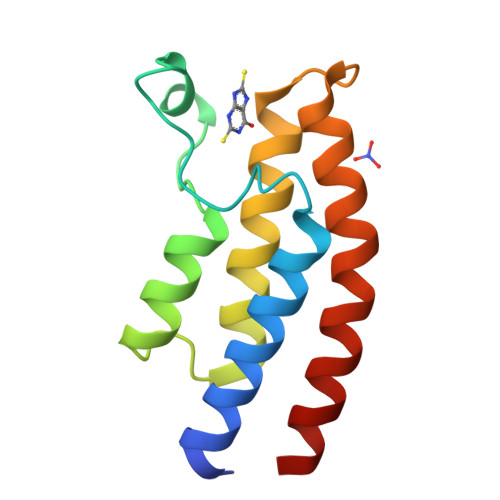
Twenty Crystal Structures of Bromodomain and PHD Finger Containing Protein 1 (BRPF1)/Ligand Complexes Reveal Conserved Binding Motifs and Rare Interactions.
Zhu, J., Caflisch, A.(2016) J Med Chem 59: 5555-5561
- PubMed: 27167503
- DOI: https://doi.org/10.1021/acs.jmedchem.6b00215
- Primary Citation of Related Structures:
5C7N, 5C85, 5C87, 5DY7, 5DYA, 5DYC, 5E3D, 5E3G, 5EM3, 5EPR, 5EPS, 5EQ1, 5ETB, 5ETD, 5EV9, 5EVA, 5EWC, 5EWD, 5EWH - PubMed Abstract:
BRPF1 plays a scaffolding role in transcription. We report on fragment screening by high-throughput docking to the BRPF1 bromodomain which resulted in six chemotypes with very favorable ligand efficiency (0.45-0.50 kcal/mol per non-hydrogen atom). Twenty crystal structures of BRPF1/ligand complexes show structural conservation in the acetyllysine binding site, common binding motifs, and unusual interactions (e.g., the replacement of a conserved water molecule). The structural information is useful for the design of chemical probes.
Organizational Affiliation:
Department of Biochemistry, University of Zürich , Winterthurerstrasse 190, CH-8057 Zürich, Switzerland.